by Dr. Frank Pleus
Reputable dentists like this Dentist in Land O’ Lakes pride themselves on using only the best materials in their treatments, and this is one of the reasons why they are so popular; clients trust that they are in safe hands with them. However, for many years there has been an ongoing controversial discussion about amalgams as dental filling material. Despite the ostensible advantages providing the population with a long lasting and easy to handle, almost undemanding teeth restoration material which at the same time shows an exalted breaking resistance and compression strength on a high cost-efficiency level we still need to see the extensive documented list of health hazards connected with amalgams, that you may find in reports on the internet and literature. There seems to be a major difficulty in confining subjective ailments and objective disease patterns, which leads to all kind of different beliefs and arguments among the experts whether amalgam should still be considered in dentistry. There is no conclusive answer except that the market of dental filling materials offers a vast variety of much more credible and unequivocally better substances in a sense of outmatching biocompatibility like high content gold alloys, ceramics and certain plastic respectively cement materials, regarding the aspect of temporarily or definite solutions.
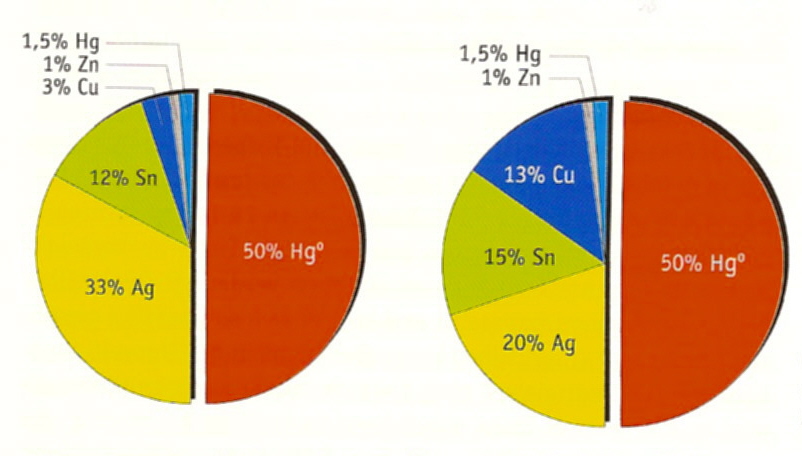
Looking at amalgams we deal with a mixture/alloy of approx. 50% liquid mercury (Hg) and 50% powder made from zinc (Zn), tin (Sn), copper (Cu), silver (Ag) and traces of other heavy metals like palladium (Pd), platinum (Pt), antimony (Sb) etc. The during early years used so called gamma-2-amalgams did also consist of 50% mercury but included a higher copper fraction causing the material to be prone to corrosion with less abrasiveness. This is why critics think that there could be a higher mercury release in comparison to the from most dental associations around the world favoured non-gamma-2 respectively gamma-2-free-amalgams, which contain 51.5% mercury, a minimum of 20% silver, a maximum of 16% tin and a maximum of 15% copper with a maximum of 1% zinc.
Boyd Haley (Boyd E. Haley PhD * 22.Sept 1940 Greensburg, Indiana; professor of the University of Kentucky Lexington and chair of the chemistry department since 1996) and his team have shown in 2007 that 1 cm2 of amalgam surface releases approx. 15yg of mercury at average during 24 h without mechanical stress, which we naturally find within the oral cavity. Mercury acts as a cell poison binding to body protein. This is why mercury as a soluble compound should be considered as toxic. At room temperature mercury exists as a liquid and gradually vapors to a gaseous condition. These mercury vapors reach mucous membranes within the lungs and thus enter the body being in a later stage stocked within the central nervous system and kidneys, where they can unfold their toxic impact.
The saliva of people with amalgams contains 3 times higher values of methylmercury, a high toxic, organic compound of mercury, than people with no amalgam fillings. Toxification with methylmercury has been shown in the surrounding of the city of Minamata on the Japanese coast, when in the mid 50s local authorities found out about an environmental catastrophe reaching world wide attention many years after due to uncontrolled sewage water dumping of a chemical corporation named Chissso. Even first denying that there might be any relation to a high percentage of people living in this area suffering out of a sudden from extreme tiredness, exhaustion, head- and body aches, partial palsy, psychosis and even coma in later stages, the company had to admit after governmental investigation that the ocean dumping of methylmercuryiodide lead to a dramatic accumulation of methylmercury within the sea algae and fish, consumed preferably by the population living on the sea shores. According to present day evaluation 17.000 people had been damaged by methylmercury in a more or less extensive way; but only 2265 people by the year 2000 had been acknowledged officially to be victims of Minamata-disease meanwhile more than 3000 people have died in the meantime.
The effects of an even more complex organic compound of mercury, the dimethylmercury, had been demonstrated in the tragic death of Karen Wetterhahn (*16.10.1948; + 08.06.1997), who used to work as a professor for chemistry at the Dartmouth College in Hanover (New Hampshire) dealing with the toxicity of metals. While she was testing the pathogenic impact of toxic metals on cells she sustained a work injury whereby dimethylmercury dripped on her protecting latex-glove diffusing through and being reabsorbed by her skin. 10 months later she was complaining about severe dizziness and headache. According to the results of a scientific laboratory study that involved analyzing a cell sample using laboratory incubators, the mercury concentration of her blood accounted for more than 80 times the toxic threshold value. Despite chelation therapy, she fell into a coma and died of acute mercury toxification. Being injured at work can have serious consequences sometimes (this is the same if you had an accident at home though). If you have been injured at work then it is really important that you get things checked over. You might also benefit by getting legal aid from a company like The House of Workers Compensation, particularly if the injury isn’t your fault.
Boyd Haley showed in some of his studies that most of the pathogenic bacteria and fungus in the mouth, within tooth pockets, from dead teeth or infected jaw bone might be able to transform the mercury, organically bound, to complex forms of dimethylmercury with a even higher toxic impact; According to his opinion these mechanisms might be the reason for the development of so called “oral Supertoxins”. This can be avoided by paying attention to other interference fields and foci within the oral cavity.
Animal experiments on sheep with radioactively marked mercury have shown that after 28 days of amalgaminsertion an accumulation of mercury could be demonstrated within the jaw bone and oral soft tissues of the amalgam exposed animals. Pregnant sheep could demonstrate perfectly the pass-through effect mercury has on the placenta. The radioactively marked mercury could be detected in the fetus as well. After removal of the mercury fillings they could show high accumulation of mercury within the sheep’s intestinal tract, bone marrow, kidneys and adrenals; the main organs for detoxification. This is why we think that there shouldn’t be any kind of removal without protective drainage therapy.
Most of the patients we have at Paracelsus Clinic didn’t have their fillings for 28 days to carry along; most of them had been exposed to mercury for more than 20 up to 50 years. Even among those that had been rehabilitated by replacing all the amalgam fillings they had, we frequently find amalgam particle within soft tissue, jaw bone and below gold crowns and/or prosthetic bridgework. If you’re starting to experience pain in your jaw from recent filling or in general, you may need treatment of temperomandibular disorders. Don’t worry, it’s very common in patients with pain in their jaw, face, head and neck.
The clinical symptoms we observe in people suffering from Amalgam toxification are mainly vegetative, neurological and skin respectively mucous membranes affecting disorders.
Clinical symptoms of Amalgam Intoxification:
| General Symptoms | Vegetative NS |
| Trembling, Tremor mercuralis | |
| Insomnia | |
| Anorexia | |
| Chronic fatigue, low energy, easy exhaustion, general oscitacy | |
| Reduced reactivity, feeling of weakness | |
| Concentration and thinking disorders |
| Head | Neurological |
| Headaches, migraines, facial neuralgias | |
| Burning tongue, glossopyrosis, dry mouth, aphthous ulcers | |
| Dental impressions on sideways of tongue | |
| Metallic taste in mouth | |
| Neckaches | |
| Dizziness | |
| Eye disorders, impaired vision |
| Skin | Skin / Mucous Membrane |
| Hair loss, dull (non-luster/less) hair | |
| Pruritus, skin eruptions, dirty gray facial skin | |
| Localized erythemas | |
| Perspiration | |
| Chronic Cystitis | |
| Colitis, Stomatitis, Bronchitis |
Regarding threshold values for amalgam it’s similar like with other environmental toxins, it’s impossible to judge about the real body burden relying on blood, urine, hair or saliva analyses. Drasch et al. were able to demonstrate within a population of gold miners sicken with amalgam intoxification, that 70% showed no elevated mercury concentration in their blood or urine values. Autopsystudies illustrate the same phenomena. Some cadavers might show low urine mercury content with high tissue mercury load, meanwhile others might show high urine mercury content with low kidney tissue burden and vice versa. Looking at the table the values for mercury within urine and kidney tissue do not correlate positively. This means there is no reason for thinking that the mercury levels within the urine predict the mercury kidney tissue content. There are bodies that show 0.3 nanogram mercury within the urine meanwhile the kidney tissue shows 350 ng Hg per gram. Others show 2 ng Hg/ml within the urine meanwhile the kidney tissue had just 150 ng Hg/g to offer.
This corresponds with the findings of Boyd Haley. The sheep showing the radioactively marked mercury after 28 days of amalgam insertion load to certain organs did not show any elevated mercury levels within their blood- and urin evalues.
It’s not necessarily the mercury that’s excreted by the body through blood, urine, hair or feces which can be made responsible for the toxicity, it’s rather the mercury that stays within the organs and cells. At the same mercury exposure people with high mercury values within the blood, hair and urine might be healthier and having less mercury stored within the organs than people that don’t show any mercury within the mentioned biomarkers. The detoxification capacity differs individually and is partially related to genetical polymorphism of detoxification enzymes of phase I and II, and at the same time depending on the accumulated total toxic burden someone might carry around and the supply with nutrients, that help to eliminate toxins.
Even with lead there is no threshold value existing that can exclude any damage.
This is why it’s not miraculous that e.g. autistic children do have eight times less mercury levels in their first hair cut after birth than healthy children – despite they had been exposed to much higher values of mercury via placentar transfer and other early childhood exposures (motherly amalgams and thimerosal from vaccinations)
Looking at the table for mercury values within the first haircut of healthy and autistic children according to motherly amalgam fillings during pregnancy, healthy children show increasing mercury values in case their mother show ascending amounts of amalgam fillings. Autistic children don’t show this effect and might not be able to excrete the motherly given mercury load via hair and blood and it’s presumably stored in the brain where it leads to tremendous damage. It’s quite understandable why the lower the mercury levels within the hair were the more pronounced the autism was.
From this perspective the following questions seem easy to answer:
Why do those patients with high mercury values in their urine (after DMPS Test) recover most often from their symptoms after replacement therapy for amalgams and correct drainage therapy? And why did those children with the highest mercury values showed the best development status? – it seems that those patients showing high mercury levels in their urine, blood and hair, have high capacity of drainage meanwhile low values are suspect for storing the mercury within the organism.
Experts, who deny any correlation of professional mercury exposure and disease development but definitely see a link between subjectively experienced symptoms referred as being caused by amalgams/mercury and psychosomatic pathologies often argue, that according to the half-life of mercury every exposure of mercury to the body should be eliminated within 20-50 days. This would imply, that the mercury once having entered the body by whatever way, has left totally within a relatively short period of time without leaving any residues all naturally happening. Scientific data and clinical experience draws different conclusions. Mercury can have a very individual half-life especially within the brain. Opitz et al. demonstrated this in 1996 with a case where a 41 year old worker who was exposed to mercury vapors in 1974 suffering from a so called “organic psychosyndrome” the following 16 years before his final death: at the beginning the mercury values within the urine had been high as expected, after four weeks of chelation therapy the values dropped down to normal. But for the patient the symptoms remained. He was still suffering from enormous tiredness, inner restlessness, burning sensations within the intestines etc. Up until 1986 several forensic experts claimed that the mercury content of the body must have been normal since 1976 and thus the ongoing symptoms are not relateable to the mercury exposure; especially since the provocations testings with chelators didn’t show any elevated values within the patient’s urine. The patient died in 1990 at the age of 57 from lung cancer without being able to work again. The autopsy showed mercury values within:
Cerebellum (2.190 ng/g); Occiput (1.090 ng/g); Thalamus (1.010 ng/g); Kidneys (1.650 ng/g); Lung (600ng/g); Thyroid (250 ng/d).
In comparison in vitro experiments show that 0.2 ng/g can already cause damage to a cell. Neither the natural way of elimination nor chelationtherapy was obviously able to eliminate the mercury in total. With a half-life of 80 days the mercury must have been eliminated within 2 years. This tragic patient is not an isolated case once happening among millions.
There are beliefs among experts that mercury contamination derives mainly from food chain (vegetables, fruit, potatoes, fish) and at the very end of the list amalgams. Experiments on dead bodies from Guzzi et al. 2006 demonstrate that people with amalgams show a twice up to 12 times higher mercury deposit within their organs than people without amalgams. Furthermore it was demonstrated that there was a ten times higher mercury deposit found within the brains of people showing 12 and more amalgam fillings in comparison to cadavers that had less than three amalgam fillings (even not been mercury free). Remarkable with this study was also, that those with the higher amount of mercury in their brains had not only more amalgam fillings but did die conspicuously often from suicide. That a mercury exposure can lead to suicidal thoughts or even to an elevated suicide rate has been shown in studies done with swedish dentists (Arnetz BB, Horte LG, Hedberg A, Malker H: Suicide among Swedish dentists. A ten year follow-up study. Scand J soc Med 1987; 15 (4): 243-6.) Throughout the years there had been several other studies done that describe the correlation between amalgam using practitioners and their staff showing frequently neuro-psychological abnormalities and measurable disturbance of memory. Some even claim a higher coincidence of pathological muscle biopsies among amalgam using dentists and staff. 85% of the dentists showed elevated values of Coproporphyrine within their urine, which indicates a dysfunction of their Haem synthesis. The same was shown for 53% of children with autism.
Haem and subgroups (a,b,c,d,o,l,m,s) have many essential functions:
- It’s vitally important for detoxification as part of all P450-enzymes
- As part of the breathing chain within the mitochondriae it’s essential for the oxidative energyproduction
- Within the red blood cells it’s important for the transportation of oxygen and carbon dioxide elimination
- Drains beta-amyloid from the brain which can cause Alzheimer disease
The average mercury concentration within brain tissue of people with amalgams (more than 12 fillings) add up to 300 ng mercury per gram brain tissue. This equals a concentration of 1.5 yMol. Among patients with less than 4 amalgam fillings a concentration of 0.1 yMol was found. Within the kidneys of people with amalgams (more than 10 fillings) they found a mean of 2.52 yMol of mercury concentration; meanwhile people with less than three fillings showed a mean value of 0.27 yMol. (121,33)
Whether the mercury concentrations measured in people with different amounts of amalgam fillings were toxic could be demonstrated in animal and in-vitro cell testings.
We need to consider that the mercury used in these tests had been inorganic mercury, mercurychloride, which is less accessible to the body’s cells than mercury vapor or organically bound methylmercury; because once the mercury vapor has reached the cell’s inside it’s oxidized to an inorganic mercury ion and can hardly escape which leads to structural damage. It’s therefore one of the most toxic forms of mercury.
The literature cited below could demonstrate that mercury concentrations of 0.1 yMol can already lead to neural damage (axonal degeneration and development of neurofibrills), which can be observed in many neurological illnesses. Concentrations of 0.18 yMol mercury lead to a secretion of beta-amyloid (a protein that accumulates within the brain and constrains brain function), elevated oxidative stress and a hyperphosphorylation of the tau-protein; and that’s exactly what happens in Alzheimer’s. Concentrations of 0.27 – 2.7 yMol lead to measurable interference with human immune cell production, liver-, kidney- and brain cell function. There was also a potentized toxic effect of mercury visible if silver, tin, copper, zinc and other heavy metals e.g lead had been around.
Still amalgams do have a big lobby around the world and paradoxically it’s either save in human teeth or on a hazardous waste deposit (as soon it’s no longer in human teeth). It remains to be seen whether the resolution of the UNO environmental ministers made in February 2009 will be transferred to abolish amalgam as a dental filling material within the next two years.
As literature shows mercury can lead to neurological changes typical for Alzheimer Disease. Low concentrations of mercury were able to destroy microtubuli, that had been responsible for the mechanical stabilization of the cell, its external form, for the active motion of the cell and for transportation processes within the cell. Mercury even in low concentrations could provoke elevation of oxidative stress (cell ageing), beta-amyloid secretion and development of neurofibrillar tangles (tangles = Neurofibrills showing structural changes).
But there are also genetical risk factors for Alzheimer’s: the apolipoprotein E (Apo E). It exists in different variations: Apo E2, Apo E3 and Apo E4. Every human posseses two of these Apolipoproteins (one from the father and one from the mother). People with two Apo E2 have a 50% reduced risk to acquire Alzheimer’s disease; those that possess two Apo E4 have a 15 times elevated risk to acquire Alzheimer’s. People with two Apo E3 have a normal risk to get sick. The only difference between these Apolipoproteins is the possession of sulfurgroups, that can protect the brain from toxins. The Apolipotproteins consist of 299 aminoacids and the cysteine contains sulfurgroups. Meanwhile:
Apo E2- shows at position 112 and 158 a Cysteine aminoacid (this is why people with two Apo E2 have 4 sulfurgroups in total to avoid heavy metal damage)
Apo E3 – possesses at position 112 a cysteine, at position 158 a arginine aminoacid
Apo E4 – possesses at position 112 and at position 158 an arginine aminoacid and does not show a protective cysteine aminoacid. This is why these people with a genetic deficit of Cysteine react more sensitive to heavy metals and develop more often Alzheimer’s than the other groups.
Africans, who stay with their traditional nutrition, rarely suffer from Alzheimer’s despite they more often show the bad Apo E4 in comparison to the white population. As soon as they begin to live in industrialized states with different eating habits; mainly sugar and refined starches, they more often suffer from cavities and receive amalgams; at the same time their risk for Alzheimer’s rises.
5% of all adults in the USA and Europe suffer from an autoimmune disease. Autoimmune diseases can be summarized as a disturbance of the immune system against autogenic tissue leading to inflammation in diverse areas and organs of the body. At the university of Dusseldorf Ernst Gleichmann and his team found out that in experiments with mice the immunitary reaction against an antigen can be influenced through gold- and mercury salts. Whilst without heavy metals the antigen was attacked by T-cells correctly, after a treatment with gold- and/or mercury salts the immune system no longer reacted appropriately to the antigen. There is quite good reason to believe that these mechanisms are also responsible for the development of autoimmune diseases.
The inhalation of mercury vapors have to be found to cause ulcerative Colitis. The replacement of amalgams and following correct drainage treatments lead to a decrease of antibodies in Hashimoto disease. Even rheumatoid arthritis can be presumed to be one of the autoimmune diseases caused by heavy metals or at least as an important co-factor. Some publications reveal a correlation between amalgams and the development of multiple sclerosis and the course of the disease. One of the analysis illustrates a 3.9 times higher risk for people with a median amount of amalgam fillings to develop multiple sclerosis in comparison to people with no amalgam in their mouths. There also seems to be a correlation between the incidence of cavities and the consequently usage of amalgams with the occurrence of multiple sclerosis. Some MS epidemics have been brought up by acute exposition with mercury vapors or lead. Inorganic mercury showed in animal testings loss of myelin sheaths (by decrease of swannon cells) and antibody increase against the myelin basic protein. Swedish investigations showed that liquor of MS patients had 7.5 higher concentrations of mercury in comparison to non-MS patients. MS patients that did have an amalgam removal after MS had been manifested showed significantly better blood values, less depression and less MS symptoms as well as less relapses. On the other hand amalgam removal was able to cause MS attacks, this means that mobilization of heavy metals and mercury from body depots might be responsible for relapses since the immune cell is repeatedly exposed to the heavy metal in case no proper drainage or precaution had been considered. This is why we are in favor for a maximally protected, gentle removal without any irksome means in combination with an effective detoxification protocol.
Mutter et al. points out that there is great evidence for inter-correlations of mercury exposure of the mother and the child to develop autism. The main source for mercury for a newborn child comes from the amalgam fillings of its mother having first intrauterine contact since mercury isn’t stopped by the placenta barrier. In case there is a genetic disposition a second contact with heavy metals e.g. through vaccination during early childhood may cause the total picture of autism. Some parents observe that their child looses some of their abilities right after infant vaccinations even those they used to handle already in an appropriate manner like walking or talking.
It was shown experimentally that the toxicity of mercury can be elevated through other vaccinationadditives like Neomycin and aluminium. At the same time there seems to be augmentable measelviruses playing an important role in the development of autism, since these viruses could be increasingly detected in brain and lymphatic tissue of autistic children.
Also the usage of antibiotics or Acetaminophen (Paracetamol) in early childhood might be a riskfactor, since antibiotics or Acetaminophen can lead to a lower excreation rate of mercury.
Nutritional factors seem to be decisive as well; there is evidence that a possible toxic impact of glutamate and aspartame elevates the toxicity of heavy metals.
Amyotrophic lateral sclerosis (ALS) – Lou Gehrig’s disease
As a progressive and rather fatal neurodegenerative disease caused by the degeneration of motor neurons, those nerve cells in the central nervous system that control voluntary muscle movement. Named after Lou Gehrig, a famous New York Yankees baseball player, who was diagnosed with the disease in 1939. The best known person today diagnosed with ALS is Stephen Hawking. The disorder causes muscle weakness and atrophy throughout the body including the muscles necessary for breathing. From school medicine perspective riluzol might be able to cause a 3 monthly elongation of survival time since this medication blocks the neurotransmitter glutamat, which has been made responsible for neural tissue damage and breakdown. According to scientific studies the disease begins with elevated oxidative stress within the nervous system. The elevated oxidative stress is supposed to lead to an elevated excretion of the neurotransmitter Glutamat, which in higher quantities is able to damage nerve cells. The real reason for the oxidative stress within the central nervous system are controversially discussed. But only 2% of all ALS patients do show a mutation of the Superoxiddismutase 1 Gene, which would also lead to elevated oxidative stress. On the other hand ALS patient most often have less reduced Glutathione available due to polymorphisms of the glutathion-s-transferase (another important detoxification enzyme). This way there is less glutathione available for the detoxification of mercury.
It becomes a vicious circle. Elevated mercury levels are responsible for elevated oxidative stress, which elevates the glutamate amount. At the same time mercury reduces the amount of glutathione, which normally should be used for reducing the oxidative stress. It’s very obvious that the mercury including other heavy metals can become very destructive for neural tissue.
There had been animal testings showing that mercury accumulates in motor neurons and elevates the oxidative stress which leads to nerve cell damage. Within mice simple mercury vapors were able to destroy motor neurons. Besides mercury it seems that lead is also involved in ALS development.
Mercury burden lower the capability to produce serotonin resp. the precursor 5 hydroxytryptophane within the brain. The experience with ALS patients shows that they have difficulties to detox heavy metals. Even on provocation with DMPS they don’t show elevated levels of heavy metals in their urine samples. At least within animal experiments it was shown that heavy metal detoxification is worthwhile treating ALS.
The job-related mercury burden elevates the risk for Parkinson’s disease 8 times. This had been shown in dentists and dental staff using amalgams in their daily practice with higher mortality rates due to dementia and Parkinson’s disease. Parkinson’s patients did have a significantly higher amount of amalgam fillings in their mouths and showed higher values of mercury within their tissues. Those patients following a detoxification protocol did report an amelioration of their symptoms. There is evidence that even other metals like manganese, copper, iron and lead might be responsible for the development of Parkinson’s disease. The same counts for pesticides. As with Alzheimer’s it seems like the existence of Apolipoprotein E4 elevates the risk for Parkinson’s, since Apo E4 can’t eliminate heavy metals properly due to the missing cysteine as sulfur group donator.
Working with dental filling materials always means compromising. There is no material that fulfills all criteria we would like to be fulfilled not endangering the body and being as durable and lasting as the original tooth.
What I want you to understand is that we might want to live with certain materials even though not perfect to all circumstances, but that we also should definitely avoid certain materials due to their possible hazards and impacts on the human body.
As we have learned there is: amalgam, palladium, platin, titanium, chromium-cobalt-molybdenum alloys (so called:steel prothesis; some of the older dentists might know this material; still existing in ordinary practices because of it’s cost effectiveness), metal or steel posts, soldering oxid compounds that might be part of a construction;
On the other hand we have non-metal materials: adhesives, bonding agents, plastics as filling material, prothesis, veneer, temporaries or endodontic material: guttapercha which is supposed to be radioactive, root filling pastes that contain normally: formaldehyde, methanal, antibiotics, cortisone; ceramic paints for individualized tooth appearance at manufactured plastic teeth (e.g.Vita). we all know that these plastics components do polymerize within the mouth for months up to years that polymer exposure should be avoided wherever possible.